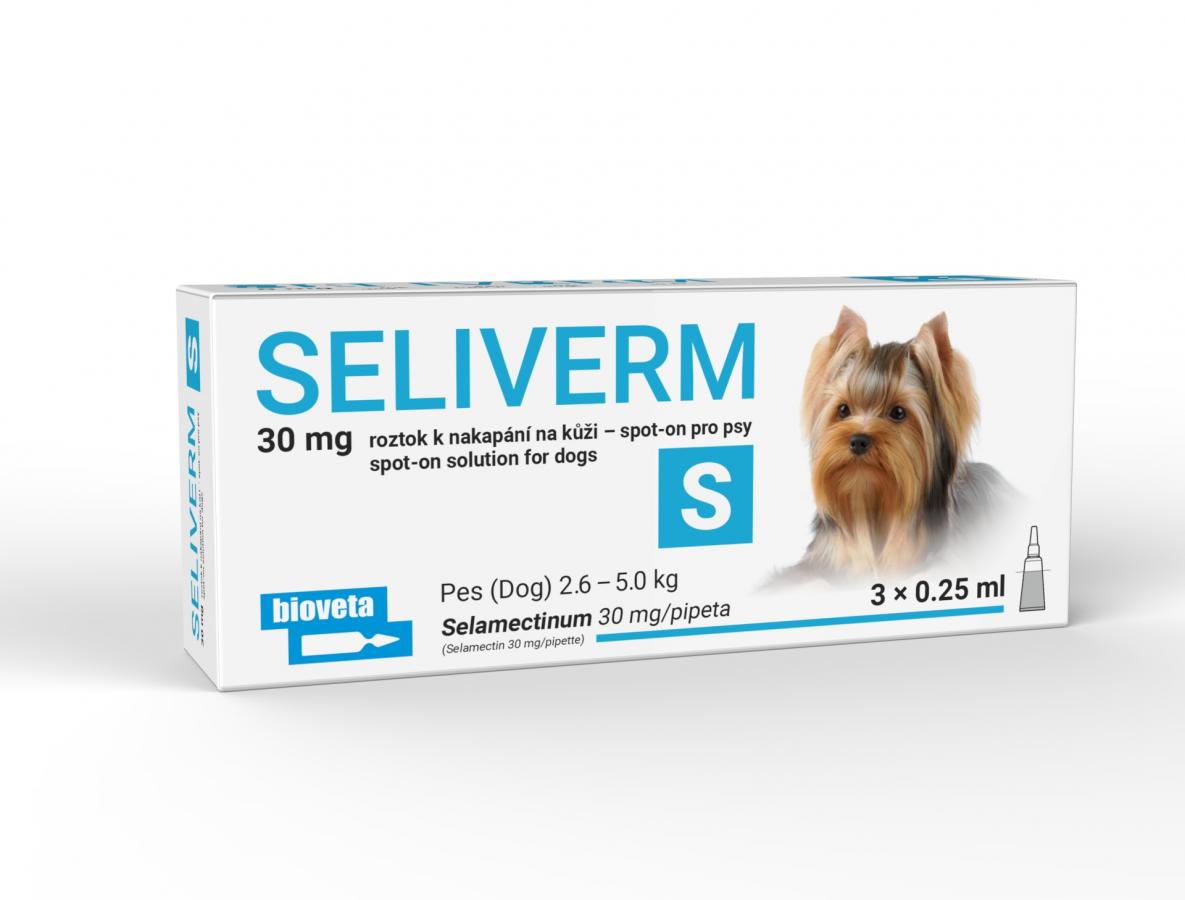
SELIVERM 30 mg spot-on solution for dogs S
Homepage Products Veterinary products SELIVERM 30 mg spot-on solution for dogs S
This product is designed for application on the skin and is effective against both external and internal parasites. It contains selamectin as the active ingredient, which helps prevent and treat parasitic infections in dogs, cats, puppies, and kittens.
| type of preparative: | Antiparasitics |
|---|---|
| target species animals: | Dog |
Composition
Each single-dose pipette contains:
Active substance:
Selamectin 30 mg
Excipients:
Butylhydroxytoluene (E 321) 0.8 mg/ml
A clear, colourless to yellow or yellow-green solution.
Target species
Dogs.
Indications for use
- Treatment and prevention of flea infestations caused by Ctenocephalides spp. for one month following a single administration. This is as a result of the adulticidal, larvicidal and ovidical properties of the product. The veterinary medicinal product is ovicidal for 3 weeks after administration. Through a reduction in the flea population, monthly treatment of pregnant and lactating animals will also aid in the prevention of flea infestations in the litter up to seven weeks of age. The product can be used as a part of a treatment strategy for flea allergy dermatitis and through its ovicidal and larvidical action may aid in the control of existing environmental flea infestations in areas to which the animals have access.
- Prevention of heartworm disease caused by Dirofilaria immitis with monthly administration. Selamectin may be safely administered to animals infected with adult heartworms, however, it is recommended, in accordance with good veterinary practice, that all animals 6 months of age or more living in countries where a vector exists should be tested for existing adult heartworm infections before the administration of the veterinary medicinal product. It is also recommended that dogs should be tested periodically for adult heartworm infections, as an integral part of a heartworm prevention strategy, even when the product has been administered monthly. This veterinary medicinal product is not effective against adult D. immitis.
- Treatment of ear mites (Otodectes cynotis).
- Treatment of biting lice infestations (Trichodectes canis)
- Treatment of sarcoptic mange (caused by Sarcoptes scabiei)
- Treatment of adult intestinal roundworms (Toxocara canis)
Contraindications
Do not use in animals under 6 weeks of age.
Special warnings
Special warnings:
Animals may be bathed 2 hours after treatment without loss of efficacy.
Do not apply when the animal’s hair coat is wet.
Special precautions for safe use in the target species:
This veterinary medicinal product is to be applied to the skin surface only. Do not administer orally or parenterally.
Keep treated animals away from fires and other sources of ignition for at least 30 minutes or until the hair coat is dry.
For ear mite treatment, do not apply directly to the ear canal.
It is important to apply the dose as indicated to minimise the quantity that the animal can lick off. If significant licking does occur, a brief period of hypersalivation may rarely be observed in cats.
Special precautions to be taken by the person administering the veterinary medicinal product to animals:
People with sensitive skin or known allergy to veterinary medicinal products of this type should handle the veterinary medicinal product with caution.
Do not smoke, eat or drink while handling the product.
Wash hands after use and wash off any product in contact with the skin immediately with soap and water. If accidental eye exposure occurs, flush the eyes immediately with water and seek medical advice immediately and show the package leaflet or the label to the physician.
Avoid direct contact with treated animals until the application site is dry. On the day of treatment, children must not handle treated animals and the animals should not be permitted to sleep with their owners, especially children. Used applicators should be disposed of immediately and not left within the sight or reach of children.
This product is highly flammable; keep away from heat, sparks, open flames or other sources of ignition.
Special precautions for the protection of the environment:
Do not allow treated animals to bathe in water courses until at least two hours after treatment administration.
Pregnancy and lactation:
Can be used in pregnant and lactating dogs.
Fertility:
Can be used in breeding dogs.
Interaction with other medicinal products and other forms of interaction:
In extensive field testing no interactions between selamectin and routinely used veterinary medicinal products or medical or surgical procedures were observed.
Overdose:
No side effects were observed after 10 times the recommended dose. The veterinary medicinal product was administered at 3 times the recommended dose to cats and dogs infected with adult heartworms and no undesirable effects were observed. The veterinary medicinal product was also administered at 3 times the recommended dose to breeding male and female cats and dogs, including pregnant and lactating females nursing their litters and at 5 times the recommended dose to ivermectin-sensitive Collies, and no undesirable effects were observed.
Major incompatibilities:
Not applicable.
Adverse events
Dogs:
|
Rare (1 to 10 animals / 10 000 animals treated): |
application site hair changes1 |
|
Very rare (<1 animal / 10 000 animals treated, including isolated reports): |
neurological signs (including seizures)2 |
1Local temporary clumping of the hair at the application site and/or an occasional appearance of a small quantity of a white powder which typically disappear within 24 hours of treatment administration and does not affect either the safety or efficacy of the veterinary medicinal product.
2Reversible as with other macrocyclic lactones.
Dosage for each species, routes and method of administration
Spot-on use.
Apply to the skin at the base of the neck in front of the shoulder blades.
The veterinary medicinal product should be administered as a single application of a single dose delivering a minimum of 6 mg selamectin/kg. When concurrent infestation or infections in the same animal are to be treated, only one application of the recommended 6 mg/kg dose should be administered at any one time. The appropriate length of the treatment period for individual parasites is specified below.
Administer in accordance with the following table:
|
Dogs (kg) |
Pipette label |
mg of selamectin dispensed |
|
2.6 – 5.0 |
S |
30 |
|
5.1 – 10.0 |
M |
60 |
|
10.1 – 20.0 |
L |
120 |
|
20.1 – 40.0 |
XL |
240 |
|
40.1 – 60.0 |
XXL |
360 |
|
> 60 |
|
Appropriate combination of pipettes |
Flea treatment and prevention
Following administration of the veterinary medicinal product, the adult fleas on the animal are killed, no viable eggs are produced, and larvae (found only in the environment) are also killed. This stops flea reproduction, breaks the flea lifecycle and may aid in the control of existing environmental flea infestations in areas to which the animal has access.
For the prevention of flea infestations, the veterinary medicinal product should be administered at monthly intervals throughout the flea season, starting one month before fleas become active. Through a reduction in the flea population, monthly treatment of pregnant and lactating animals will aid prevention of flea infestations in the litter up to seven weeks of age.
For use as part of a treatment strategy for flea allergy dermatitis the veterinary medicinal product should be administered at monthly intervals.
Prevention of heartworm disease
The veterinary medicinal product may be administered year-round or at least within one month of the animal’s first exposure to mosquitoes and monthly thereafter until the end of the mosquito season. The final dose must be given within one month after the last exposure to mosquitoes. If a dose is missed and a monthly interval between dosing is exceeded then immediate administration of the veterinary medicinal product and resumption of monthly dosing will minimise the opportunity for the development of adult heartworms. When replacing another heartworm preventive veterinary medicinal product in a heartworm disease prevention programme, the first dose of the veterinary medicinal product must be given within a month of the last dose of the former medication.
Treatment of roundworm infections
A single dose of the veterinary medicinal product should be administered.
Treatment of biting lice
A single dose of the veterinary medicinal product should be administered.
Treatment of ear mites
A single dose of the veterinary medicinal product should be administered. Loose detritus should be gently removed from the external ear canal at the time of treatment. A further veterinary examination 30 days after treatment is recommended as some animals may require a second treatment.
Treatment of sarcoptic mange
For complete elimination of the mites, a single dose of the veterinary medicinal product should be administered for two consecutive months.
Advice on correct administration
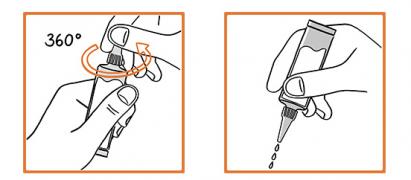
Remove the pipette from its protective package. Hold the pipette with the neck up and turn the cap 360°. Do not remove the cap as shown in the picture. Part the animal's fur at the back of the neck to expose a small area of skin. Apply the pipette tip directly to the skin. Squeeze the pipette several times to empty its contents. Application of a larger volume can cause itching in dogs and cats, and reddening or hair loss may occur. Avoid contact between the product and your fingers.
Withdrawal periods
Not applicable.
Special storage precautions
Keep out of the sight and reach of children.
Do not store above 25 °C. Store in the original package in a dry place.
Do not use this veterinary medicinal product after the expiry date which is stated on the carton and the label after Exp. The expiry date refers to the last day of that month.
Special precautions for disposal
Medicines should not be disposed of via wastewater or household waste.
This veterinary medicinal product should not enter water courses as selamectin may be dangerous for fish and other aquatic organisms.
Use take-back schemes for the disposal of any unused veterinary medicinal product or waste materials derived thereof in accordance with local requirements and with any applicable national collection systems. These measures should help to protect the environment.
Ask your veterinary surgeon or pharmacist how to dispose of medicines no longer required.
Classification of veterinary medicinal products
Veterinary medicinal product subject to prescription.
Marketing authorisation numbers and pack sizes
Pack sizes: 3 or 6 single-dose pipettes packed individually in a sealed sachet in cardboard box.
Not all pack sizes may be marketed.